Medical device firm TechMah has received 510(k) approval from the U.S. Food and Drug Administration (FDA) for its artificial intelligence (AI)-based jaw surgery software system.
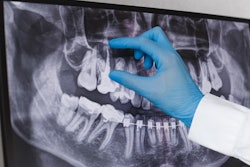
The company’s tmCMF solution includes surgeon review tool software and instruments, including guides, anatomical models, and dental splints, for use in trauma and reconstruction procedures in the jaw and midface. The instruments are created based on a patient’s computed tomography and dental scans data. The product's commercial release is expected in the third quarter of 2024.